

Updated: 2024-06-06

Recently, Professor Pang Siping, Professor Li Shenghua, Associate Professor Chen Wenxing from the Beijing Institute of Technology (BIT) and Associate Professor Zhang Liang from Tsinghua University collaborated to make important advancements in the preparation of asymmetrically coordinated dual-atom iron catalysts and their efficient electrocatalytic application for Oxygen Reduction Reaction.
The findings, titled "Precisely Constructing Charge-Asymmetric Dual-Atom Fe Sites Supported on Hollow Porous Carbon Spheres for Efficient Oxygen Reduction," were published in the top-tier journal Energy & Environmental Science (Impact Factor: 32.5) (Article DOI: 10.1039/D4EE01309C). BIT is the first research institution, with doctoral students Li Yaqiong and Wei Zihao from the BIT School of Materials Science & Engineering and postdoctoral fellow Luo Xuan from Tsinghua University as co-first authors.
Hydrogen fuel cells occupy a crucial position in China's clean energy and sustainable development strategy and have become a research hotspot in recent years. However, the sluggish kinetics of the cathodic ORR reaction has become a key factor limiting the improvement of power density and energy conversion efficiency of hydrogen fuel cells. To overcome this challenge, researchers are committed to designing catalysts with rational structures to lower the reaction barrier and accelerate the kinetics of ORR. Against this background, the team of Professor Pang Siping, Professor Li Shenghua, Associate Professor Chen Wenxing from BIT and Associate Professor Zhang Liang from Tsinghua University has made significant progress. They successfully prepared a charge-asymmetric dual-atom ORR catalyst (Fe2-S1N5/SNC) using hollow carbon spheres as carriers by controlling the morphology of the carbon carrier and the coordination environment of the metal active site. The morphology characterization of the catalyst is shown in Figure 1. In this catalyst, nitrogen-doped hollow carbon spheres serve as an ideal carrier for active sites, significantly shortening the path for substances to reach the active sites during the ORR process. Through X-ray absorption spectroscopy (XAS) techniques, the structure of the catalyst's active sites was deeply analyzed, confirming its unique charge-asymmetric characteristics, as shown in Figure 2. Furthermore, density functional theory (DFT) calculations revealed that the charge-asymmetric Fe dual-atom sites in the catalyst Fe2-S1N5/SNC exhibit more suitable adsorption energy for the ORR intermediate product OH*, facilitating the desorption of OH* and improving the reaction kinetics, as shown in Figure 3. In 0.1 mol·L-1 HClO4 solution, the half-wave potential of Fe2-S1N5/SNC is 0.829 V, close to that of commercial Pt/C catalysts, as shown in Figure 4. This result provides new insights for the design of highly active and low-cost metal catalysts, particularly regarding the precise construction of catalytic reaction active sites.
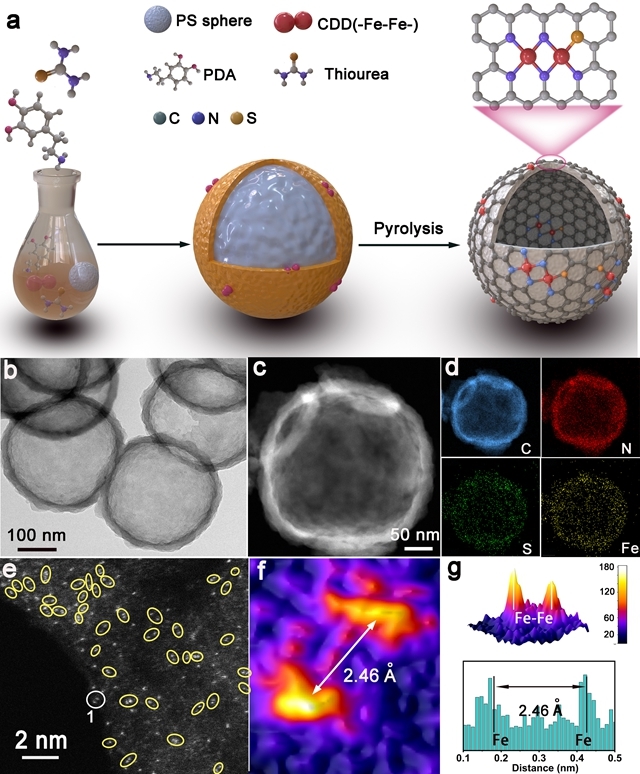
Figure 1. Synthesis strategy and morphology characterization of the asymmetric catalyst Fe2-S1N5/SNC [Photo/bit.edu.cn]
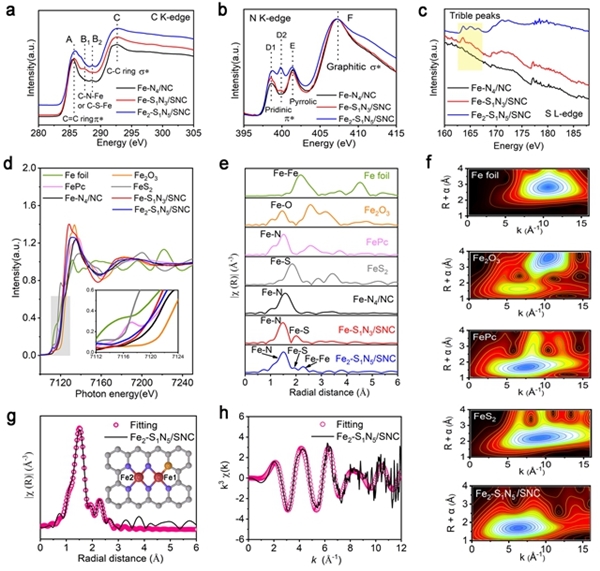
Figure 2. XAFS spectrum analysis of the asymmetric catalyst Fe2-S1N5/SNC [Photo/bit.edu.cn]
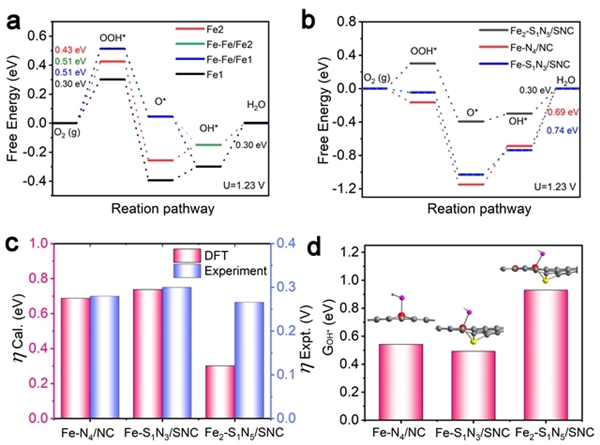
Figure 3. Theoretical calculation results of the asymmetric catalyst Fe2-S1N5/SNC [Photo/bit.edu.cn]
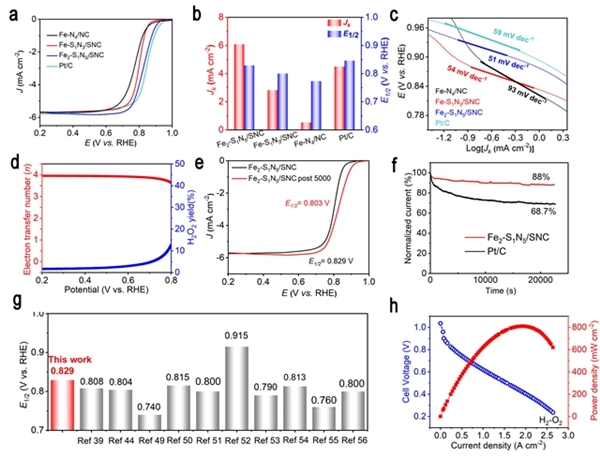
Figure 4. ORR test results of the asymmetric catalyst Fe2-S1N5/SNC [Photo/bit.edu.cn]
Paper link: https://pubs.rsc.org/en/content/articlelanding/2024/ee/d4ee01309c
This research project was supported by the National Natural Science Foundation of China, the Research Fund for Excellent Youth Teachers of BIT, the National Key Laboratory of Intelligent Green Vehicles and Smart Transportation, and the Scientific Research Start-Up Fund and the High-Performance Computing Center of Tsinghua University. Special thanks to Teacher Zhang Fang from the Analytical Testing Center of BIT for her support in transmission electron microscopy characterization.














